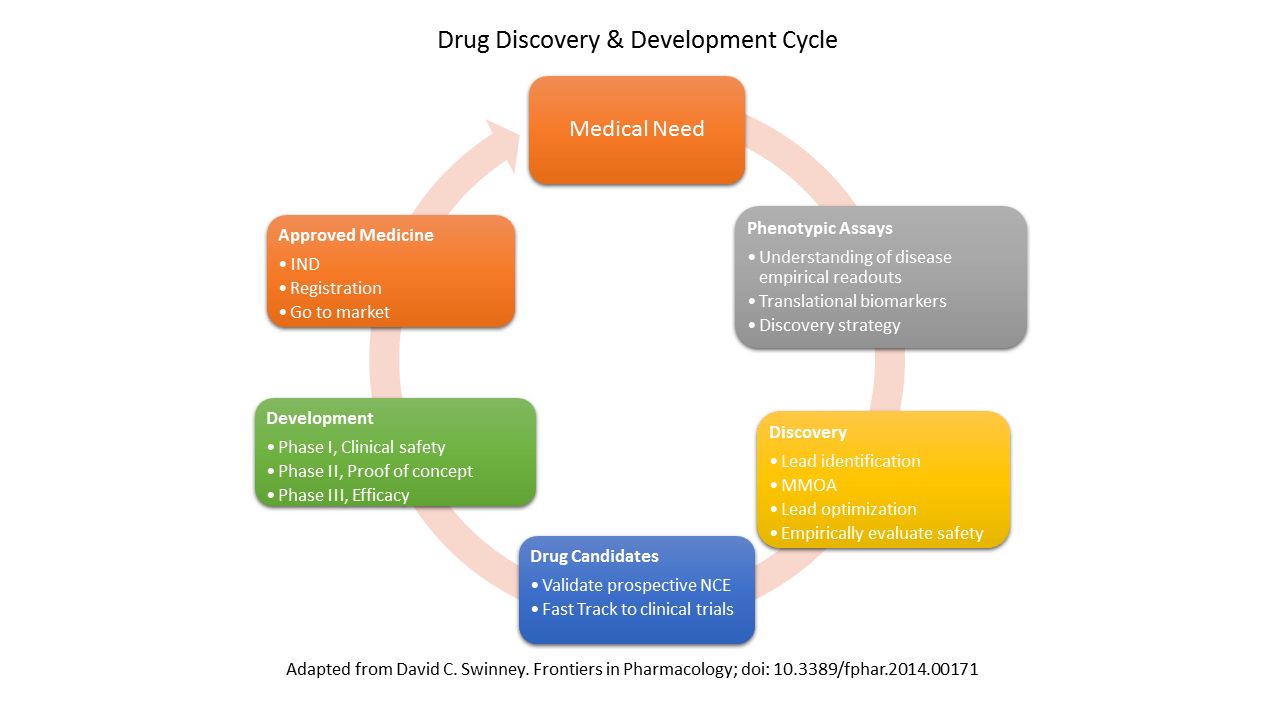
Phenotypic assays are tools essential for drug discovery.
Phenotypic assays have different types of endpoints depending on the goals
- empirical endpoints for basic research to understand the underlying biology that will lead to identification of translation biomarkers,
- empirical endpoints to identify undesired effects related to toxicity of drug candidates,and
- knowledge-based endpoints (biomarkers) for drug discovery which ideally are translational biomarkers that will be used to identify new drug candidates and their corresponding molecular mechanisms of action
The value of phenotypic assays is increased through effective alignment of phenotypic assay endpoints with the objectives of the relevant stage in the drug discovery and development cycle.
There is a great need for validated translational biomarkers to guide drug discovery in order to identify safe and effective medicines prior to clinical evaluation.
This is key to decreasing attrition and increasing productivity of pharmaceutical research.
The reality is that the more relevant the system is to physiology the better it will predict the clinical success.
Associated with this complexity is the feasibility of obtaining predictive information.
Phenotypic assays that translate effectively to human disease will always be required for the reasons described above,including the ability to identify an optimal MMOA and derisk safety.
Reference:
1. DavidC.Swinney. The value of translational biomarkers to phenotypic assays. Frontiers in Pharmacology; doi: 10.3389/fphar.2014.00171







 Your message was successfully sent. Thank You!
Your message was successfully sent. Thank You!